COVID-19 Vaccine Statistics
United States
258.7 M (78%)
of 331 M vaccinated
World
5.190 B (66%)
of 7.794 B vaccinated
Boosters
U.S. 103.6 M (31.1%)
World 1.996 B (25.3%)
Global Vaccination Status
U.S. Vaccination Status
Vaccine Efficacy, Safety, and Cost Profile
Special Reports on Vaccine Development
Epidemiology
Critical News
FDA authorizes first COVID booster for children ages 5 to 11 (NPR) - May 17, 2022
The Food and Drug Administration Tuesday authorized the first COVID-19 vaccine booster for children ages 5 to 11. The authorization makes all children in that age group who received their second shot at least five months ago eligible to receive a third shot of the Pfizer-BioNTech vaccine. Until now, only children ages 12 and older and adults were eligible for a booster.
The companies requested the authorization based on a small study that the companies and FDA said demonstrated a third shot is safe and can significantly boost antibody levels, countering waning immunity and providing added protection against the virus, including the more contagious omicron variant.
U.S. approves second Covid-19 booster for people 50 and older (STAT) - March 29, 2022
The Food and Drug Administration on Tuesday authorized second Covid-19 boosters for people 50 years and older for those who want them. People 50 and older are now eligible for another shot of either the Pfizer-BioNTech and Moderna mRNA vaccines four months after their last dose, the FDA said. The FDA on Tuesday also authorized second boosters for people with certain immunocompromising conditions.
Health officials cited data from Israel showing that second boosters increased antibody levels, while other studies from Israel have shown that the shots increased protection against death during the country’s Omicron wave. Much of that data is considered preliminary, and it’s only been a few months since those doses started going into arms. Pfizer and BioNTech also said they submitted data to the FDA showing some waning of effectiveness three to six months out from the first booster shots.
While some people will eagerly roll up their sleeves once more, it does not seem there is huge immediate demand for another shot, particularly just months after the initial booster campaign. Fewer than half of people who are eligible for boosters and who had their primary series of shots have received an initial booster. Among those 65 and older, just two in three vaccinated people have had a booster. A primary reason the United States had a much worse Omicron wave than some European countries — in terms of hospitalizations and deaths, not just infections — was because of the country’s relatively low booster rate among seniors, who needed the third shot to maintain strong protection against a virus as mutated as Omicron. Some countries have already authorized second boosters for older adults. The United Kingdom is offering another round of shots to people 75 and older this spring, with a wider campaign potentially planned for later on.
Sanofi and GSK to seek regulatory authorization for COVID-19 vaccine (Sanofi) - February 23, 2021
In the VAT08 Phase 3 primary series trial, two doses of the Sanofi-GSK vaccine in seronegative populations demonstrated:
* 100% efficacy against severe COVID-19 disease and hospitalizations
* 75% efficacy against moderate or severe COVID-19 disease
* 57.9% efficacy against any symptomatic COVID-19 disease, in line with expected vaccine effectiveness in today’s environment dominated by variants of concernThe public health relevance of the refrigerator temperature-stable adjuvanted protein-based Sanofi-GSK vaccine is strongly supported by the induction of robust immune responses and a favorable safety profile in multiple settings. In participants who had received a primary series of an already authorized mRNA or adenovirus vaccine, the Sanofi-GSK booster vaccine induced a significant increase in neutralizing antibodies of 18- to 30-fold across vaccine platforms and age groups. When the Sanofi-GSK vaccine was used as a two-dose primary series followed by a booster dose, neutralizing antibodies increased 84- to 153-fold compared to pre-boost levels.
Moderna receives full U.S. FDA Approval for COVID-19 Vaccine Spikevax (Moderna, FDA) - January 31, 2022
The U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older.
The updated analyses to determine effectiveness of Spikevax included 14,287 vaccine recipients and 14,164 placebo recipients 18 years of age and older who did not have evidence of SARS-CoV-2 infection prior to receiving the first dose. The data used for the analyses were accrued before the Omicron variant emerged. These data demonstrated that Spikevax was 93% effective in preventing COVID-19, with 55 cases of COVID-19 occurring in the vaccine group and 744 COVID-19 cases in the placebo group. The vaccine was also 98% effective in preventing severe disease.
FDA Authorizes Merck’s Covid-19 Pill for At-Home Treatment (WSJ, FDA) - December 23, 2021
U.S. regulators cleared use of a Covid-19 pill from Merck & Co. and partner Ridgeback Biotherapeutics LP, the latest easy-to-use therapy that infected people can take to keep out of the hospital. Merck, based in Kenilworth, N.J., has agreed to provide the U.S. government with 3.1 million courses of the drug for about $2.2 billion. The U.K. cleared molnupiravir for use in November.
Merck’s molnupiravir is approved for the treatment of mild-to-moderate COVID-19 in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate. Molnupiravir is available by prescription only and should be initiated as soon as possible after diagnosis of COVID-19 and within five days of symptom onset.
The primary data supporting this EUA for molnupiravir are from MOVe-OUT, a randomized, double-blind, placebo-controlled clinical trial studying molnupiravir for the treatment of non-hospitalized patients with mild to moderate COVID-19 at high risk for progression to severe COVID-19 and/or hospitalization. Of the 709 people who received molnupiravir, 6.8% were hospitalized or died within this time period compared to 9.7% of the 699 people who received a placebo. Of the people who received molnupiravir one died during the follow-up period compared to nine people who received placebo.
Pfizer’s COVID-19 Pill Is Authorized in U.S. (WSJ, FDA) - December 22, 2021
U.S. health regulators cleared use of a Covid-19 pill from Pfizer Inc., the first drug that newly infected patients can now take at home to stay out of the hospital. The U.S. government has agreed to pay $5.29 billion to purchase 10 million treatment courses that Pfizer will deliver by the end of next year. Pfizer said it aims to make 120 million treatment courses next year, up from its previous projection of 80 million. The pill’s key advantage over most existing treatments is that patients can easily take them at home, unlike antibody therapies or the antiviral Veklury that usually require administration at a clinic or hospital.
Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) is approved for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about 88 pounds) with positive results of direct SARS-CoV-2 testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is available by prescription only and should be initiated as soon as possible after diagnosis of COVID-19 and within five days of symptom onset.
The primary data supporting this EUA for Paxlovid are from EPIC-HR, a randomized, double-blind, placebo-controlled clinical trial studying Paxlovid for the treatment of non-hospitalized symptomatic adults with a laboratory confirmed diagnosis of SARS-CoV-2 infection. Paxlovid significantly reduced the proportion of people with COVID-19 related hospitalization or death from any cause by 88% compared to placebo among patients treated within five days of symptom onset and who did not receive COVID-19 therapeutic monoclonal antibody treatment. In this analysis, 1,039 patients had received Paxlovid, and 1,046 patients had received placebo and among these patients, 0.8% who received Paxlovid were hospitalized or died during 28 days of follow-up compared to 6% of the patients who received placebo.
Pfizer’s Covid pill remains 89% effective in final analysis (STAT) - December 14, 2021
Paxlovid, Pfizer’s pill to treat Covid-19, retained its 89% efficacy at preventing hospitalization and death in the full results of a study of 2,246 high-risk patients, the company said Tuesday. In the study of high-risk patients, called EPIC-HR, 5 of 697 patients who received a five-day course of Paxlovid were hospitalized or died, compared to 44 of 682 who received a placebo. There were no deaths in the Paxlovid group and 9 in the placebo group. Adverse events occurred at similar rates between the placebo and Paxlovid groups, and patients on Paxlovid were less likely to have a severe problem or to stop taking the drug due to a perceived side effect. Patients in the study were considered high risk because they were not vaccinated and had at least one characteristic or underlying medical condition that increased their risk of Covid-19. These could include being over 65, being overweight, or having cardiovascular disease.
Pfizer also reported results from a second study in adults with Covid at normal risk of developing severe disease, a group that included vaccinated people. That study failed to meet its main goal, of increasing the sustained alleviation of self-reported symptoms, at an interim analysis; the study is continuing. But Pfizer said that there was a decrease in hospitalization in that group, too, although numbers were small. The study of patients who were at lower risk, called EPIC-SR, showed that 2 of 333 patients who received a five-day course of Paxlovid were hospitalized compared to 8 of 329 who received placebo. The results were similar in a second analysis, Pfizer said, but barely missed statistical significance. Rates of adverse events were similar between the drug and placebo.
First case of Omicron coronavirus variant identified in the U.S. (STAT) - December 1, 2021
The Covid-19 case was identified by the California and San Francisco health departments in a person who had traveled to South Africa and returned on Nov. 22, the Centers for Disease Control and Prevention said in a release. The individual, who was fully vaccinated with the Moderna shot but had not received a booster, had mild symptoms and has since recovered, federal and local officials said. The person has been isolating since testing positive on Nov. 29. All close contacts have tested negative thus far. […] Some two dozen countries, from the United Kingdom to Australia to Israel, have already reported cases, many in travelers.
Pfizer and BioNTech investigating new COVID-19 variant (CNBC) - November 26, 2021
“We understand the concern of experts and have immediately initiated investigations on variant B.1.1.529,” the companies said. Pfizer and BioNTech said they expect more data from lab tests in two weeks at the latest. Pfizer and BioNTech said they can adapt their mRNA vaccine within six weeks and start shipping batches within 100 days if an escape variant is identified.
Several European and Asian nations have suspended flights from southern Africa in response to the variant. The United Kingdom suspended flights on Thursday from six countries in the region, and the European Commission – the European Union’s executive body – told all 27 member states to halt travel from southern Africa.
New Coronavirus Variant Arrives in Europe, Sets Off Global Fears of Restrictions (WSJ) - November 25, 2021
Belgian authorities said that one case of the B.1.1.529 variant has been recorded in the country. The person traveled from abroad, was unvaccinated and hadn’t been infected with COVID-19 previously.
South Africa Raises Alarm Over New Coronavirus Variant (WSJ) - November 25, 2021
Researchers first detected B.1.1.529 on November 12. Since then, the variant has driven an exponential rise in COVID-19 infections in the country, with about 4-times as many infections as 2 weeks ago. B.1.1.529 is now responsible for around 90% of cases in South Africa’s most populous province, Gauteng. It has also been detected in neighboring Botswana and in a South African traveler in Hong Kong.
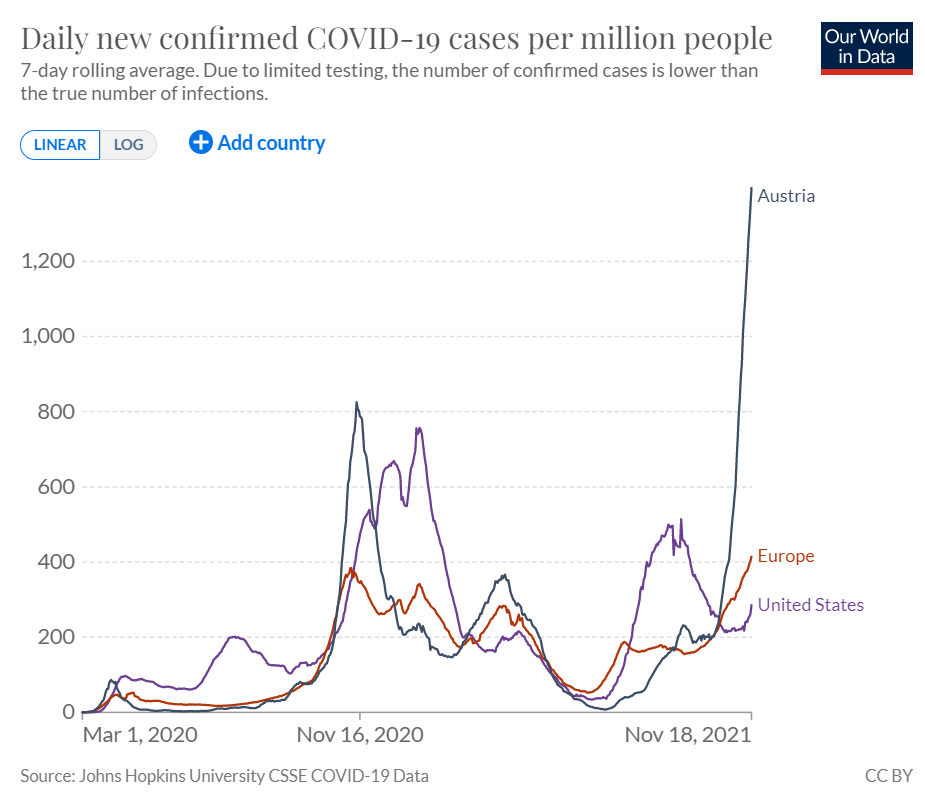
Austria re-imposes a full Covid lockdown and makes vaccination mandatory (CNBC) - November 19, 2021
Austria will enter a fourth national lockdown on Monday as Covid-19 cases continue to surge, becoming the first country in Western Europe to impose stringent measures this fall. The country’s unvaccinated are already barred from leaving their homes for non-essential purposes. The lockdown would last for a maximum of 20 days, but would initially be put in place for 10 days.
On Thursday, Austria recorded 15,145 new cases of Covid-19, setting a new record high for daily positive tests. Hospitalizations, deaths and the number of Covid patients in ICU are also rising fast in Austria. Around 65% of Austria’s population has been fully vaccinated against the virus, which Chancellor Alexander Schallenberg has previously described as “shamefully low.” The country has the second-lowest vaccination rate in Western Europe after Liechtenstein.
FDA expands emergency authorization for Covid-19 booster shots to all adults (STAT) - November 19, 2021
Today’s action expands the use of booster doses of both vaccines to include all individuals 18 years of age and older at least six months after completion of the primary vaccination series of the Moderna COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine or at least two months after completion of primary vaccination with the Janssen COVID-19 Vaccine.
Though the Biden administration announced its intention to offer boosters to all vaccinated Americans in August, two groups of outside experts who advise the FDA and the CDC were initially unconvinced of the need — and irked by a White House decision that circumvented normal processes for this type of a decision.
Data from the CDC suggest that people who are eligible for booster shots are getting them, with 37.3% of fully vaccinated people 65 and older having already received a booster. Vaccine uptake in that age group is reasonably high; 86.2% of Americans 65 and older are fully vaccinated.
Pfizer Seeks Emergency Use Authorization for Novel COVID-19 Oral Antiviral Candidate (Pfizer) - November 16, 2021
Pfizer is seeking EUA for PAXLOVID based on positive results from the EPIC-HR interim analysis, which enrolled non-hospitalized adults aged 18 and older with confirmed COVID-19 who are at increased risk of progressing to severe illness. The data demonstrated an 89% reduction in risk of COVID-19-related hospitalization or death from any cause in patients treated with PAXLOVID compared to placebo within three days of symptom onset, with no deaths in the treatment group. Similar results were seen with within five days of symptom onset. Treatment-emergent adverse events were comparable between PAXLOVID (19%) and placebo (21%), most of which were mild in intensity. At the recommendation of an independent Data Monitoring Committee, and in consultation with the U.S. FDA, Pfizer ceased further enrollment into the study due to the overwhelming efficacy demonstrated. Rolling submissions have commenced in several countries including in the United Kingdom, Australia, New Zealand and South Korea, with planned submissions to other regulatory agencies around the world to follow.
Pfizer has begun and will continue to invest up to approximately $1 billion of its own funds to support the manufacturing and distribution of this investigational treatment candidate. Additionally, Pfizer has signed a voluntary licensing agreement with the Medicines Patent Pool (MPP) to help expand access, pending regulatory authorization or approval, in 95 low- and middle-income countries that account for approximately 53% of the world’s population.
Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study (Pfizer) - November 05, 2021
The scheduled interim analysis showed an 89% reduction in risk of COVID-19-related hospitalization or death from any cause compared to placebo in patients treated within three days of symptom onset (primary endpoint); 0.8% of patients who received PAXLOVID™ were hospitalized through Day 28 following randomization (3/389 hospitalized with no deaths), compared to 7.0% of patients who received placebo and were hospitalized or died (27/385 hospitalized with 7 subsequent deaths). The statistical significance of these results was high (p<0.0001). Similar reductions in COVID-19-related hospitalization or death were observed in patients treated within five days of symptom onset; 1.0% of patients who received PAXLOVID™ were hospitalized through Day 28 following randomization (6/607 hospitalized, with no deaths), compared to 6.7% of patients who received a placebo (41/612 hospitalized with 10 subsequent deaths), with high statistical significance (p<0.0001). In the overall study population through Day 28, no deaths were reported in patients who received PAXLOVID™ as compared to 10 (1.6%) deaths in patients who received placebo.
FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age (FDA) - October 29, 2021
Effectiveness: Immune responses of children 5 through 11 years of age were comparable to those of individuals 16 through 25 years of age. In addition, the vaccine was found to be 90.7% effective in preventing COVID-19 in children 5 through 11.
Safety: The vaccine’s safety was studied in approximately 3,100 children age 5 through 11 who received the vaccine and no serious side effects have been detected in the ongoing study
The Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age is administered as a two-dose primary series, 3 weeks apart, but is a lower dose (10 micrograms) than that used for individuals 12 years of age and older (30 micrograms). In the U.S., COVID-19 cases in children 5 through 11 years of age make up 39% of cases in individuals younger than 18 years of age. According to the CDC, approximately 8,300 COVID-19 cases in children 5 through 11 years of age resulted in hospitalization. As of Oct. 17, 691 deaths from COVID-19 have been reported in the U.S. in individuals less than 18 years of age, with 146 deaths in the 5 through 11 years age group.
Moderna Announces Positive Top Line Data from Phase 2/3 Study of COVID-19 Vaccine in Children 6 to 11 Years of Age (Moderna) - October 25, 2021
KidCOVE is a randomized, observer-blind, placebo-controlled expansion study to evaluate the safety, tolerability, reactogenicity and effectiveness of two 50 µg doses of mRNA-1273 given to healthy children 28 days apart. The study population is divided into 3 age groups (6 to <12 years, 2 to <6 years, and 6 months to <2 years). Today Moderna reports on the 6 to under 12 years of age cohort.
This cohort enrolled 4,753 participants who were 6 to less than 12 years of age. In the trial, the SARS-Cov-2-neutralizing antibody geometric mean ratio (GMR) comparing the response in children to the response in young adults from the Phase 3 COVE study was 1.5 (95% Cl: 1.3, 1.8), with a seroresponse rate of 99.3%, representing a difference of 0.6% (95% CI: -2.8%, 2.8%) to the Phase 3 benchmark. These results demonstrate strong immune response in this cohort of children one month after the second dose and met the co-primary immunogenicity endpoints for 6 to less than 12 years olds in KidCOVE.
mRNA-1273 was generally well tolerated with a safety and tolerability profile generally consistent with the Phase 3 COVE study in adolescents and adults. The majority of adverse events were mild or moderate in severity. The most common solicited adverse events were fatigue, headache, fever, and injection site pain.
Moderna plans to submit these data to the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA) and other global regulators in the near term.
Pfizer-BioNTech Covid-19 Vaccine for Kids Was Safe and 90.7% Effective, Companies Say (WSJ) - October 22, 2021
The companies released new data Friday showing the vaccine was 90.7% effective at preventing symptomatic Covid-19 in a study of [children 5 to 11 years old], information that health authorities will likely factor as they weigh whether to authorize the shots for use. The vaccine was found to be safe and tolerable, the companies said in a document they submitted to the Food and Drug Administration, which the agency posted online Friday morning. […] Each child received two doses three weeks apart, and each shot contained 1/3 of the dosage used for adolescents and adults. The vaccine induced neutralizing antibody levels in younger children that were comparable to those seen in people ages 16 to 25 who served as a control group in the study, the companies said. […] Researchers didn’t find any cases of heart-inflammation conditions including myocarditis in children in the study. The companies, however, said the study was too small to detect this potential risk. Health authorities have said there is an increased risk of myocarditis with Covid-19 vaccines from Pfizer and Moderna Inc., particularly in males under 30 years.
The documents are being posted ahead of a meeting scheduled for Tuesday of the FDA’s Vaccines and Related Biological Products Advisory Committee, will meet to review the evidence of the vaccine’s safety and effectiveness in children, and vote to recommend whether the FDA should authorize the use. If the vote is in favor, FDA authorization could follow within days. And if the Centers for Disease Control and Prevention subsequently signs off, millions of younger children could get a Covid-19 vaccine for the first time. There are more than 28 million children ages 5 to 11 in the U.S., according to the American Academy of Pediatrics.
Pfizer-BioNTech Covid-19 Booster Shot Was 95.6% Effective in Large Trial, Companies Say (WSJ) - October 21, 2021
A third dose of the Covid-19 vaccine from Pfizer/BioNTech was found in a large study to be highly protective against symptomatic Covid-19. Researchers found 109 cases of symptomatic Covid-19 among study subjects who received a placebo shot, compared with five cases in people who took the vaccine, resulting in 95.6% efficacy. NOTE: earlier data released by Pfizer during their Q2 Earnings report stated that the 2-dose primary series declined to 84% in preventing symptomatic disease after six months from a peak of 96%. In the study, the companies enrolled more than 10,000 people who were 16 years and older and had received the two-dose shot. Pfizer and BioNTech enrolled subjects in the U.S., Brazil and South Africa who had been part of the late-stage study that led to the vaccine’s original authorization. Half of the subjects got a booster dose, and the rest took a placebo.
The additional dose was safe and tolerable, and consistent with what was known about the vaccine. The study was carried out while the highly contagious Delta variant was prevalent suggesting the booster helps protect against the contagious strain.
FDA Takes Additional Actions on the Use of a Booster Dose for COVID-19 Vaccines (FDA) - October 20, 2021
Today, the U.S. Food and Drug Administration took action to expand the use of a booster dose for COVID-19 vaccines in eligible populations. The agency is amending the emergency use authorizations (EUA) for COVID-19 vaccines to allow for the use of a single booster dose as follows:
> Moderna Booster: The use of a single booster dose of the Moderna COVID-19 Vaccine that may be administered at least 6 months after completion of the primary series to individuals: (1) >65 years old, (2) 18-64 years of age at high risk of severe COVID-19, (3) 18-64 years of age with frequent institutional or occupational exposure to SARS-CoV-2.
> J&J Booster: The use of a single booster dose of the Janssen (Johnson and Johnson) COVID-19 Vaccine may be administered at least 2 months after completion of the single-dose primary regimen to individuals 18 years of age and older.
> Mix & Match Boosters: The use of each of the available COVID-19 vaccines as a heterologous (or “mix and match”) booster dose in eligible individuals following completion of primary vaccination with a different available COVID-19 vaccine.
FDA Delays Moderna Covid-19 Vaccine for Adolescents to Review Rare Myocarditis Side Effect (WSJ) - October 15, 2021
The Food and Drug Administration is delaying a decision on authorizing Moderna Inc.’s Covid-19 vaccine for adolescents to assess whether the shot may lead to heightened risk of a rare inflammatory heart condition, according to people familiar with the matter. After four Nordic countries strengthened their stances against giving Moderna vaccines to younger adults last week, the FDA has been taking another look at the risk of the condition, known as myocarditis, among younger men who took Moderna’s vaccine, especially compared with those who received the vaccine from Pfizer/BioNTech. So far, the regulators haven’t determined whether there is an elevated risk, the people said. The delay could be several weeks, but the timing is unclear, one of the people said.
FDA advisory panel votes 19-0 to endorse booster dose of J&J vaccine (STAT) - October 15, 2021
A Food and Drug Administration advisory panel voted Friday that booster shots should be made available to people who have received the Johnson & Johnson Covid-19 vaccine. Unlike the authorizations for boosters for the Pfizer/BioNTech and Moderna vaccines, no restrictions were put on the J&J booster. The panel effectively said that the J&J vaccine, like the other vaccines, requires two doses to be effective.
Merck Asks FDA to Authorize Promising Covid-19 Pill (WSJ) - October 12, 2021
Merck and Ridgeback Biotherapeutics today announced that Merck has submitted an Emergency Use Authorization (EUA) application to the U.S. Food and Drug Administration (FDA) for molnupiravir, an investigational oral antiviral medicine, for the treatment of mild-to-moderate COVID-19 in adults who are at risk for progressing to severe COVID-19 and/or hospitalization.
The filing comes shortly after data from a late-stage study showed that the antiviral drug, molnupiravir, cut the risk of hospitalization or death by about 50% in high-risk people with mild to moderate Covid-19. […] The Merck-Ridgeback pill, if authorized, would be the first oral antiviral for Covid-19. A course of treatment is 40 pills, eight daily for five days, started within five days of showing symptoms. […] Merck plans to manufacture 10 million courses of treatment by the end of the year and has already begun production.
The Kenilworth, N.J.-based drugmaker has a $1.2 billion deal with the U.S. to provide 1.7 million courses of treatment, should regulators clear it for use. Merck also has said it would make molnupiravir available globally and has licensing agreements with generic drugmakers, including Dr. Reddy’s Laboratories Ltd. and Sun Pharmaceutical Industries Ltd. , to ensure the drug’s availability to low-income countries. The company also said it plans to peg pricing for molnupiravir to the wealth of the country buying it, based on World Bank criteria, to help expand access to low- and middle-income countries.
Today, we submitted an Emergency Use Authorization application to the U.S. FDA for our investigational #COVID19 #antiviral treatment. Read about today’s milestone: https://t.co/BHPzfkSw4K $MRK pic.twitter.com/y5WdTQnyHL
— Merck (@Merck) October 11, 2021
Pfizer, BioNTech seek COVID-19 vaccine approval for children 5 to 11 (Reuters) - October 7, 2021
Pfizer Inc and BioNTech have asked U.S. regulators to approve emergency use of their COVID-19 vaccine for children aged five to 11, Pfizer said in a tweet on Thursday. The U.S. Food and Drug Administration has set a date of Oct. 26 for its panel of outside advisers to meet and discuss the application, making it possible for kids to begin receiving the vaccines shortly afterwards. The vaccine is already authorized in teens aged 12-to-15 and fully approved for ages 16 and up, and has been shown to induce a strong immune response in the target age group in a 2,268 participant clinical trial, the companies said on Sept. 20.
Children currently make up about 27% of all U.S. coronavirus cases and an increasing percentage of hospitalizations, according to the American Academy of Pediatrics. That reflects the high contagiousness of the Delta variant among unvaccinated people. While kids are less susceptible to severe COVID-19, they can spread the virus to others, including vulnerable populations that are more at risk of severe illness. The two drugmakers are also testing the vaccine in children ages 2-to-5 years and children ages 6 months-to-2 years, with data expected in the fourth quarter of 2021.
EMA recommendations on extra doses and boosters (EMA) - October 4, 2021
EMA’s human medicines committee (CHMP) has concluded that an extra dose of the COVID-19 vaccines Comirnaty (BioNTech/Pfizer) and Spikevax (Moderna) may be given to people with severely weakened immune systems, at least 28 days after their second dose. The recommendation comes after studies showed that an extra dose of these vaccines increased the ability to produce antibodies against the virus that causes COVID-19 in organ transplant patients with weakened immune systems.
FDA to Hold Advisory Committee Meetings to Discuss Emergency Use Authorization for Booster Doses and COVID-19 Vaccines for Younger Children (FDA) - October 1, 2021
On Oct. 14, the committee will discuss an amendment to the emergency use authorization of the Moderna COVID-19 Vaccine for the administration of a booster dose, in individuals 18 years of age and older.
On Oct. 15, the VRBPAC will discuss amending the emergency use authorization of Johnson and Johnson’s Janssen COVID-19 Vaccine for the administration of a booster dose, in individuals 18 years of age and older.
Additionally, on Oct. 15, the committee will hear a presentation from the National Institute of Health’s National Institute of Allergy and Infectious Diseases on the heterologous use of booster doses following the primary series of the three currently authorized or approved COVID-19 vaccines.
The FDA anticipates receiving a request from Pfizer to amend its emergency use authorization to allow the use of its COVID-19 vaccine in children 5 through 11 years of age. In anticipation of the request, the FDA is moving forward with scheduling an advisory committee meeting on Oct. 26 to inform the agency’s decision-making.
The FDA intends to livestream the VRBPAC meetings on the agency’s YouTube page (Oct. 14 meeting link; Oct. 15 meeting link; Oct. 26 meeting link), which will be viewable on the agency’s Facebook and Twitter channels; the meetings will also be webcast from the FDA website.
CDC Director Dr. Rochelle Walensky endorses ACIP recommendation for a booster shot of Pfizer-BioNTech COVID-19 vaccine (CDC) - September 24, 2021
Today, CDC Director Rochelle P. Walensky, M.D., M.P.H., endorsed the CDC Advisory Committee on Immunization Practices’ (ACIP) recommendation for a booster shot of the Pfizer-BioNTech COVID-19 vaccine in certain populations and also recommended a booster dose for those in high risk occupational and institutional settings. CDC recommends:
> people 65 years and older and residents in long-term care settings should receive a booster shot of Pfizer-BioNTech’s COVID-19 vaccine at least 6 months after their Pfizer-BioNTech primary series,
> people aged 50–64 years with underlying medical conditions should receive a booster shot of Pfizer-BioNTech’s COVID-19 vaccine at least 6 months after their Pfizer-BioNTech primary series,
> people aged 18–49 years with underlying medical conditions may receive a booster shot of Pfizer-BioNTech’s COVID-19 vaccine at least 6 months after their Pfizer-BioNTech primary series, based on their individual benefits and risks, and
> people aged 18-64 years who are at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting may receive a booster shot of Pfizer-BioNTech’s COVID-19 vaccine at least 6 months after their Pfizer-BioNTech primary series, based on their individual benefits and risks.
FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations (FDA) - September 22, 2021
Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least 6 months after completion of the primary series in: (1) individuals 65 years of age and older; (2) individuals 18 through 64 years of age at high risk of severe COVID-19; and (3) individuals 18 through 64 years of age whose frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.
“The FDA amended the EUA for the Pfizer-BioNTech COVID-19 Vaccine to allow for a booster dose in certain populations such as healthcare workers, teachers and day care staff, grocery workers and those in homeless shelters or prisons, among others,” said Acting FDA Commissioner Janet Woodcock, M.D.
To support the authorization for emergency use of a single booster dose, the FDA analyzed safety and immune response data from a subset of participants from the original clinical trial of the Pfizer-BioNTech COVID-19 Vaccine. In addition, consideration was given to real-world data on the vaccine’s efficacy over a sustained period of time provided by both U.S. and international sources, including the CDC, the UK and Israel. The immune responses of approximately 200 participants 18 through 55 years of age who received a single booster dose approximately six months after their second dose were assessed. The antibody response against SARS-CoV-2 virus one month after a booster dose of the vaccine compared to the response one month after the two-dose primary series in the same individuals demonstrated a booster response.
J&J says second shot boosts protection for moderate-severe COVID-19 to 94% (Reuters) - September 21, 2021
Johnson & Johnson said Tuesday a second shot of its COVID-19 vaccine given about two months after the first increased its effectiveness to 94% in the United States against moderate to severe forms of the disease. That compares to 70% protection with a single dose.
[JNJ Press Release] The Phase 3 ENSEMBLE 2 study showed that another shot of the Johnson & Johnson COVID-19 vaccine given 56 days after the first provided: 100% protection against severe/critical COVID-19, 75% protection against symptomatic (moderate to severe/critical) COVID-19 globally, and 94% protection against symptomatic (moderate to severe/critical) COVID-19 in the U.S. (CI, 58%-100%). When a booster of the Johnson & Johnson COVID-19 vaccine was given two months after the first shot, antibody levels rose to four to six times higher than observed after the single shot.
Pfizer & BioNTech announce positive topline results from pivotal trial of COVID-19 vaccine in children 5 to 11 years (Pfizer) - September 20, 2021
Favorable safety profile and robust neutralizing antibody responses in children 5 to 11 years of age using a two-dose regimen of 10 µg administered 21 days apart, a smaller dose than the 30 µg dose used for people 12 and older. The antibody responses in the participants given 10 µg doses were comparable to those recorded in a previous Pfizer-BioNTech study in people 16 to 25 years of age immunized with 30 µg doses. The 10 µg dose was carefully selected as the preferred dose for safety, tolerability and immunogenicity in children 5 to 11 years of age. These are the first results from a pivotal trial of a COVID-19 vaccine in this age group.
FDA advisory panel recommends booster doses of Covid-19 vaccine only for older and high-risk Americans (STAT) - September 17, 2021
Members of the Vaccines and Related Biological Products Advisory Committee voted 16 to 2 against a proposal to administer a third dose of the vaccine developed by Pfizer and BioNTech to individuals 16 years and older. The vote to recommend a booster to people 65 years and older — as well as people who are at risk of severe COVID-19 — was 18 to 0.
The FDA is not required to follow the recommendation of its advisory committees but generally does. If the recommendation is adopted by the FDA and Centers for Disease Control and Prevention, it would put the U.S. policy on a par with countries like the United Kingdom. It was not immediately clear who would qualify as high risk; fleshing that out will likely fall to the CDC’s advisory committee, the Advisory Committee on Immunization Practices.
UK plans COVID boosters for over 50s (Reuters, UK Govn’t) - September 14, 2021
The U.K. Joint Committee on Vaccination and Immunization (JCVI) recommended on Tuesday an additional COVID-19 vaccine for fully vaccinated individuals over the age of 50 and all vulnerable people six months after their second dose. Their preference as a booster dose is to use full dose Pfizer/BioNTech COVID-19 shot or a half-dose Moderna vaccine. Vaccinations will begin next week and the NHS will contact people directly to let them know when it is their turn to get their booster vaccine. Plans for the roll-out will use the existing networks in place for the COVID-19 vaccination programme, including: (1) local vaccination services co-ordinated by primary care networks and community pharmacies or (2) vaccination centres across the country, ensuring people can access a booster dose regardless of where they live.
The latest data from Public Health England and Cambridge University shows vaccines have saved more than 112,300 lives and prevented 143,600 hospitalisations and 24 million cases in England. Yesterday, the government announced that people aged 12 to 15 in England would be offered one dose of the Pfizer/BioNTech COVID-19 vaccine from next week, following advice from the 4 UK chief medical officers.
Two outgoing FDA directors, WHO officials, and academics express doubt on the need for mRNA vaccine boosters in Lancet perspectives piece - September 13, 2021
Lancet article | Endpoints commentary | STAT article on FDA lead authors Marion Gruber & Phil Krause
On the need for a booster in the general population: A consistent finding is that vaccine efficacy is substantially greater against severe disease than against any infection; in addition, vaccination appears to be substantially protective against severe disease from all the main viral variants. […] Boosting could be appropriate for some individuals in whom the primary vaccination, might not have induced adequate protection—eg, recipients of vaccines with low efficacy or those who are immunocompromised. […] Current evidence does not, therefore, appear to show a need for boosting in the general population, in which efficacy against severe disease remains high.
On the waning efficacy of the primary vaccine series: Even if humoral immunity appears to wane, reductions in neutralising antibody titre do not necessarily predict reductions in vaccine efficacy over time, and reductions in vaccine efficacy against mild disease do not necessarily predict reductions in the (typically higher) efficacy against severe disease. This effect could be because protection against severe disease is mediated not only by antibody responses, which might be relatively short lived for some vaccines, but also by memory responses and cell-mediated immunity, which are generally longer lived. The ability of vaccines that present the antigens of earlier phases of the pandemic (rather than variant-specific antigens) to elicit humoral immune responses against currently circulating variants indicates that these variants have not yet evolved to the point at which they are likely to escape the memory immune responses induced by those vaccines. […] Although vaccines are less effective against asymptomatic disease or against transmission than against severe disease, even in populations with fairly high vaccination rates the unvaccinated are still the major drivers of transmission and are themselves at the highest risk of serious disease. Even if boosting were eventually shown to decrease the medium-term risk of serious disease, current vaccine supplies could save more lives if used in previously unvaccinated populations than if used as boosters in vaccinated populations
On the authors: Two lead authors, Marion Gruber (Director of FDA OVRR) and Phil Krause (Deputy Director of FDA OVRR), are key vaccine regulators at the FDA and announced their retirement/resignation on August 31, 2021. Their departure is speculated to have been incited by (1) pressure from the Biden administration to accelerate its review of the mRNA vaccine boosters by Pfizer & Moderna and (2) a perceived overreach by the CDC in determining whether boosters are appropriate. The viewpoint was also co-authored by WHO (note: WHO has officially called for a moratorium on boosting until the primary vax series is more widely administered globally) and other academic experts from around the world.
U.S. decision on Pfizer COVID-19 shot for kids age 5-11 could come in October (Reuters) - September 10, 2021
Top U.S. health officials believe that Pfizer's COVID-19 vaccine could be authorized for children aged 5-11 years old by the end of October, two sources familiar with the situation said on Friday. […] Pfizer has said that it would have data on children age 5-11 ready in September and planned to submit for an EUA shortly after. […]Top U.S. infectious disease expert Dr. Anthony Fauci outlined the timetable during an online town hall meeting attended by thousands of staff at the NIH on Friday, according to one of the sources. A second source familiar with the situation said that the FDA anticipated a similar timeline for Pfizer. If Pfizer submits its EUA by the end of September, and the data support its use, "by the time we get to October, the first couple of weeks of October … the Pfizer product will likely be ready," Fauci said, according to the source.
Fauci said that Moderna will likely take about 3 weeks longer than Pfizer to collect and analyze its data on children age 5-11, according to the source. He estimated that a decision on the Moderna shot could come around November, according to the source. The second source said Fauci's timeline for Moderna appeared "optimistic."
New Biden plan could mandate COVID shots or tests for two-thirds of U.S. workers (Reuters) - September 9, 2021
President Joe Biden will require all federal employees to get vaccinated against COVID-19 and the U.S. Department of Labor will issue a rule requiring businesses with more than 100 employees to have their workers vaccinated or tested weekly, officials said. […] The administration would also require vaccinations for more than 17 million healthcare workers at hospitals and other institutions that participate in Medicare and Medicaid social programs for poor, disabled and older Americans, senior administration officials said. The new vaccination requirements cover about 100 million workers, or about two-thirds of all workers in the United States, officials said.
In addition, the administration plans to ramp up testing capacity for the virus. Biden will use his authority under the Defense Production Act to spur industry to accelerate production of the tests, and big retailers including Walmart, Amazon.com, and Kroger will sell the tests at cost for the next three months to make them more affordable, the officials said.
Unvaccinated people are 29 times more likely to get hospitalized with COVID-19 (CDC) - August 24, 2021
During May 1–July 25, 2021, among 43,127 SARS-CoV-2 infections in residents of Los Angeles County, California, 10,895 (25.3%) were in fully vaccinated persons, 1,431 (3.3%) were in partially vaccinated persons, and 30,801 (71.4%) were in unvaccinated persons. On July 25, [unvaccinated people were 29.2-times more likely to be hospitalized and 4.9-times more likely to be infected than fully vaccinated people] (see graphs below). Efforts to enhance COVID-19 vaccination coverage, in coordination with other prevention strategies, are critical to preventing COVID-19–related hospitalizations and deaths.
Between May 1, 2021 (27% LA residents fully vaccinated) to July 25, 2021 (51% LA residents fully vaccinated)…lower percentages of fully vaccinated persons were hospitalized (3.2%), were admitted to an intensive care unit (0.5%), and required mechanical ventilation (0.2%) compared with partially vaccinated persons (6.2%, 1.0%, and 0.3%, respectively) and unvaccinated persons (7.6%, 1.5%, and 0.5%, respectively) (p<0.001). […] A lower percentage of deaths (0.2%, 24) occurred among fully vaccinated persons than among partially vaccinated (0.5%, seven) and unvaccinated (0.6%, 176) persons (p<0.001).
FDA Approves First COVID-19 Vaccine (FDA) - August 23, 2021
Today, the U.S. Food and Drug Administration approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization (EUA), including for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
Third Pfizer dose 86% effective in over 60s, Israeli HMO says (Reuters) - August 18, 2021
Israeli HMO Maccabi, which covers around a quarter of the country’s 9.3 million population, compared results from 149,144 people aged over 60 who received their third dose at least a week ago against those from 675,630 more who had received only two doses, between January and February. Some 37 people (0.02%) tested positive for coronavirus after their third jab, compared with 1,064 positive cases (0.16%) among those who had received only two doses, Maccabi said in a statement. […] Israel began administering third Pfizer doses [in July] to confront a surge in local infections driven by the Delta variant.
Covid-19 Booster Shot to Be Offered to People Fully Vaccinated With Pfizer, Moderna (WSJ) - August 18, 2021
The Biden administration on Wednesday called for a third Covid-19 shot starting in the fall for Americans who were fully vaccinated with the two-shot [mRNA vaccines from Pfizer or from Moderna Inc.], citing the threat from the highly contagious Delta variant and heightened concerns over data showing initial immunity wanes over time. Under the expanded plan, the U.S. would start offering the extra dose starting September 20, 2021 after the FDA authorizes it. People 65 and older and individuals in chronic-care facilities are expected to get boosters initially, along with health workers and anyone else who was vaccinated first, according to the authorities, including the leaders of the FDA, NIH, and CDC. […] Moderna said on Aug. 5 that its vaccine remains 93% effective against preventing Covid-19 disease for at least six months, but said it sees a decline in antibody levels after six months, especially against newer strains of the coronavirus including the Delta variant. […] The efficacy of the Pfizer-BioNTech vaccine protecting against symptomatic disease dropped every two months, to 84% after six months from a peak of 96% within two months of vaccination, according to data released in July by the companies. The research was collected before the more infectious and possibly more virulent Delta variant is believed to have begun its surge in the U.S.
About an additional dose of an mRNA COVID-19 vaccine (CDC) - August 12, 2021
Effective August 13, 2021, CDC recommends that people who are moderately to severely immunocompromised receive an additional dose of an mRNA COVID-19 Vaccine (Pfizer-BioNTech or Moderna) at least 28 days after the completion of the initial mRNA COVID-19 vaccine series. Available data show that these people don’t always build adequate levels of protection after an initial 2-dose primary mRNA COVID-19 vaccine series. The data also show that they may benefit from receiving an additional dose of an mRNA vaccine to develop as much protection as possible against COVID-19.
Currently, CDC is recommending that moderately to severely immunocompromised people receive an additional dose. This includes people who have: (1) active treatment for solid tumor and hematologic malignancies, (2) receipt of solid-organ transplant and taking immunosuppressive therapy, (3) receipt of CAR-T-cell or hematopoietic stem cell transplant (within 2 years of transplantation or taking immunosuppression therapy), (4) moderate or severe primary immunodeficiency (e.g., DiGeorge syndrome, Wiskott-Aldrich syndrome), (5) advanced or untreated HIV infection, (6) active treatment with high-dose corticosteroids (i.e., ≥20mg prednisone or equivalent per day), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, tumor-necrosis (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory.
FDA Authorizes 3rd Vaccine Dose for Certain Immunocompromised Individuals (FDA) - August 12, 2021
The U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) for both the Pfizer-BioNTech COVID-19 Vaccine and the Moderna COVID-19 Vaccine to allow for the use of an additional dose in certain immunocompromised individuals, specifically, solid organ transplant recipients or those who are diagnosed with conditions that are considered to have an equivalent level of immunocompromise. The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices is scheduled to meet Friday to discuss further clinical recommendations regarding immunocompromised individuals. The action does not apply to people who are not immunocompromised. […] People who are immunocompromised in a manner similar to those who have undergone solid organ transplantation have a reduced ability to fight infections and other diseases, and they are especially vulnerable to infections, including COVID-19. The FDA evaluated information on the use of a third dose of the Pfizer-BioNTech or Moderna Vaccines in these individuals and determined that the administration of third vaccine doses may increase protection in this population. These patients should be counseled to maintain physical precautions to help prevent COVID-19. In addition, close contacts of immunocompromised persons should get vaccinated, as appropriate for their health status, to provide increased protection to their loved ones. It is recommended that immunocompromised individuals discuss monoclonal antibody treatment options with their health care provider should they contract or be exposed to COVID-19. The FDA has authorized monoclonal antibody treatments for emergency use during this public health emergency for adults and pediatric patients (ages 12 and older weighing at least 40 kilograms or about 88 pounds) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progressing to severe COVID-19 and/or hospitalization. One authorized product includes use for preventative (prophylaxis) treatment after being exposed to SARS-CoV-2; however, this product is not a substitute for vaccination.
Moderna’s initial data for COVID-19 vaccine booster/3rd dose (Moderna) - August 5, 2021
Q2 Earnings PR | Q2 Earnings Call Transcript | Q2 Earnings Call Slides
NOTE: Consolidated information initially disclosed during Moderna’s Q2 Earnings release. Remarks by Stéphane Bancel (Chief Executive Officer at Moderna) and Stephen Hoge (President of Moderna; leads R&D). Data from Q2 Earnings Call Slides.
Six months durability data: In final analysis of Phase 3 COVE study data, the Moderna COVID-19 Vaccine showed 93% efficacy, with the efficacy remaining durable through six months after administration of the second dose.
Following those subjects forward to months six to eight after their second dose, what you can see is waning both for the wild-type virus although it remains detectable levels of titers but also for the variance of concern particularly beta, gamma and delta. […] At 14 days post dose 3, we saw 23-fold boosting against the wild-type strain to levels that are significantly above the level we've seen just after second dose one month post dose 2. Saw a 32-fold boosting against beta; 43 fold boosting against gamma; and 42-fold boosting against delta. Again all reaching levels that are significantly higher than previously seen. And that is very encouraging and we think confirms our selection of the prototype booster as likely to be protective against the circulating variance of concern particularly delta presently.
Earlier this year our Phase II study of mRNA-1273 was amended to offer a third dose to all interested participants six months out from their second dose of the vaccine. A total of 344 participants elected to receive that dose. And the top line results are reported here and we have a manuscript in preparation that we'll be submitting shortly.
But we believe a booster dose is likely to be necessary this fall, particularly in the face of the Delta variant. Our clinical data right now we think supports a 50-microgram mRNA-1273 booster and we see no obvious advantage for beta containing variant candidates driven both by the data I presented today and the evolving epidemiology. We're going to wait for 100-microgram data in the coming weeks to confirm the dose selection of 50 micrograms as the booster before filing.
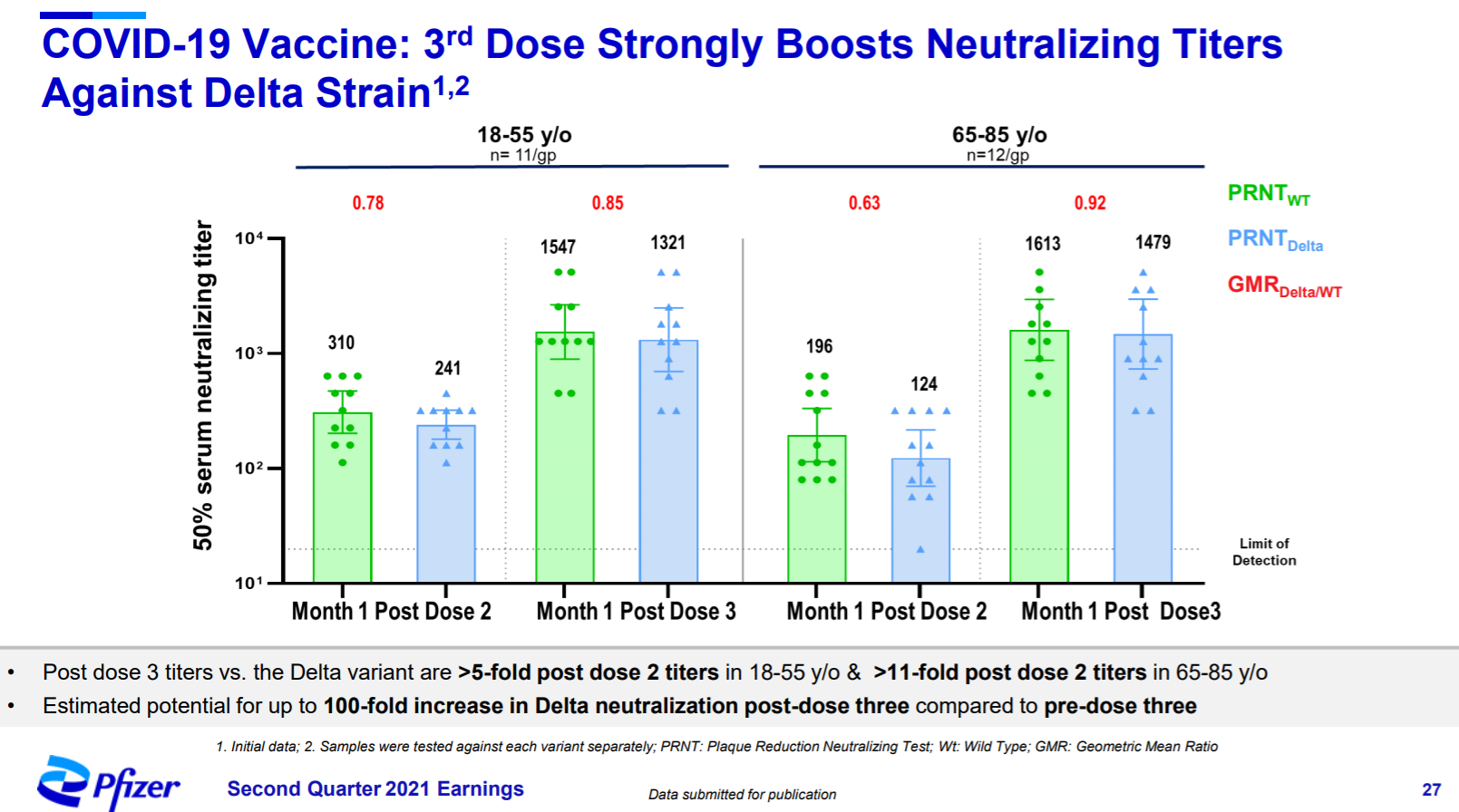
Pfizer’s initial data for COVID-19 vaccine booster/3rd dose (Pfizer) - July 28, 2021
Q2 Earnings PR | Q2 Earnings Call Prepared Remarks | Q2 Earnings Call Slides
NOTE: Consolidated information initially disclosed during Pfizer’s Q2 Earnings release. Remarks by Mikael Dolsten (Chief Scientific Officer and President, Worldwide Research, Development and Medical at Pfizer). Data from Q2 Earnings Call Slides. The efficacy of the vaccine protecting against symptomatic disease dropped every two months, to 84% after six months from a peak of 96% within two months of vaccination, a decline that may add urgency to Pfizer’s push to administer an additional dose to maintain protection (WSJ, MedRXiv).
We are in ongoing discussions with regulatory agencies regarding a potential third dose booster of the current vaccine and, assuming positive results, anticipate an FDA Emergency Use Authorization (EUA) submission as early as August. […] We observe a significant boost in neutralizing antibodies following a third dose of the current vaccine for both wild type and the Beta variant. At eight months post dose two, antibody levels start to decline from their earlier peaks. In our initial analysis, a third dose given more than six months after the second dose elicited neutralizing antibodies which are more than 5-times higher against the wild type and more than 10-times higher against the Beta variant than after two primary doses alone. The third dose elevates the neutralizing antibodies in our laboratory studies to up to 100-times higher levels post-dose three compared to pre-dose three. Just as we saw in the analysis of neutralizing antibodies from those in the original Phase 3 trial, the levels in the older population were comparable to the younger population.
The Delta variant, which is the most transmissible we’ve yet seen, is expanding rapidly worldwide and now represents approximately 83% of sequenced cases in the US. […] Here, we show new breaking data from a small number of participants that a third dose boost with the current vaccine elicited neutralizing titers that when tested against the Delta variant were more than 5-fold post-dose two in younger people and more than 11-fold post-dose two in older people. Receiving a third dose more than six months after vaccination, when protection may be beginning to wane, was estimated to potentially boost the neutralizing antibody titers in participants in this study to up to 100 times higher post-dose three compared to pre-dose three. These preliminary data are very encouraging as Delta continues to spread.
FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine (FDA) - February 27, 2021
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the third vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The EUA allows the Janssen COVID-19 Vaccine to be distributed in the U.S for use in individuals 18 years of age and older.
The totality of the available data provides clear evidence that the Janssen COVID-19 Vaccine may be effective in preventing COVID-19. The data also show that the vaccine’s known and potential benefits outweigh its known and potential risks, supporting the company’s request for the vaccine’s use in people 18 years of age and older. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness and manufacturing quality information.
The Janssen COVID-19 Vaccine is manufactured using a specific type of virus called adenovirus type 26 (Ad26). The vaccine uses Ad26 to deliver a piece of the DNA, or genetic material, that is used to make the distinctive “spike” protein of the SARS-CoV-2 virus. While adenoviruses are a group of viruses that are relatively common, Ad26, which can cause cold symptoms and pink eye, has been modified for the vaccine so that it cannot replicate in the human body to cause illness. After a person receives this vaccine, the body can temporarily make the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2.
FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine (FDA) - December 18, 2020
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the second vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The emergency use authorization allows the Moderna COVID-19 Vaccine to be distributed in the U.S. for use in individuals 18 years of age and older.
The totality of the available data provides clear evidence that the Moderna COVID-19 Vaccine may be effective in preventing COVID-19. The data also show that the known and potential benefits outweigh the known and potential risks—supporting the company’s request for the vaccine’s use in people 18 years of age and older. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness, and manufacturing quality information.
The Moderna COVID-19 Vaccine contains messenger RNA (mRNA), which is genetic material. The vaccine contains a small piece of the SARS-CoV-2 virus’s mRNA that instructs cells in the body to make the virus’s distinctive “spike” protein. After a person receives this vaccine, their body produces copies of the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2.
FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine (FDA) - December 11, 2020
The U.S. Food and Drug Administration issued the first emergency use authorization (EUA) for a vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. The emergency use authorization allows the Pfizer-BioNTech COVID-19 Vaccine to be distributed in the U.S.
The totality of the available data provides clear evidence that Pfizer-BioNTech COVID-19 Vaccine may be effective in preventing COVID-19. The data also support that the known and potential benefits outweigh the known and potential risks, supporting the vaccine’s use in millions of people 16 years of age and older, including healthy individuals. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness and manufacturing quality information.
The Pfizer-BioNTech COVID-19 Vaccine contains messenger RNA (mRNA), which is genetic material. The vaccine contains a small piece of the SARS-CoV-2 virus’s mRNA that instructs cells in the body to make the virus’s distinctive “spike” protein. When a person receives this vaccine, their body produces copies of the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2.